“Better own half of something than all of nothing.”
Menzo Havenga, President & CEO of Batavia Biosciences, lives by this quote. He is a passionate, visionary leader and acquires qualities like scientific understanding, forward-thinking, adaptability, and commitment to patient-centric outcomes. With an immense experience of 27 years, he has emerged as a distinguished figure in the biopharmaceutical field.
The Entrepreneurial Journey and Achievements
Menzo Havenga is a Molecular Virologist by training and concluded his PhD in 1992. He worked as a senior scientist in a start-up company called IntroGene to develop a platform of recombinant adenoviral vectors to treat cardiovascular diseases. IntroGene changed its name to Crucell in 2001. He worked there as Vice president of R&D from 2001 to 2005. From 2006 to 2008, he was head of R&D and led the organization of 300 employees.
At the end of 2008, he joined the TNO organization as Business unit manager for Life sciences, overseeing a 300-person organization located in Leiden and Zeist (Netherlands). Together with the board of directors at TNO, the TNO investment fund, and his business partner Chris Yallop, they launched Batavia Biosciences in 2010 as a spin-out from the TNO organization.
Menzo has co-authored over 200 publications in international scientific magazines. He has also contributed to more than 40 patents. These patents describe different methods and techniques for manufacturing cell lines and platforms, as well as viral vectors. He is also the co-inventor of the Adenovector technology used by J&J to battle the COVID-19 pandemic. As an entrepreneur and CEO, he has received numerous national and international prizes and was knighted in 2018 by the King of The Netherlands for his scientific contributions to the field.
Below is a list of some of his achievements throughout the year.
- Acquisition of Berna Biotech to Crucell (2006)
- Acquisition of SBL vaccines to Crucell (2007)
- Build & management buy-out of Batavia Biosciences from TNO (2010)
- Acquisition of Xendo Pharmaservices to Batavia Biosciences (2011)
- Lounge of Batavia Biosciences Inc in Boston USA (2012)
- Sale of Batavia Biosciences to CJ CheilJedang corporation (2021)

Bringing Innovation in Biopharmaceutical
Batavia Biosciences is a biopharmaceutical process and product development Services Company. The primary goal of it is to provide high-quality biopharmaceuticals for proof-of-concept, pre-clinical, and clinical studies, adding value to its customers. It is located at the bioscience park in Leiden, the Netherlands where it deploys a 5000 square meter R&D facility, housing about 200 employees. Its R&D services range from DNA cloning and stable cell line generation to scale-up, purification development, product characterization, and clinical manufacturing. The company also has a number of production platforms for viral vaccines and viral vectors that it offers to its customers, including research- and GMP-grade vector systems AAV, LV, MV, AdV, VSV, SINV, etc) and virus seeds (polio, Measles, Rubella, etc).
By the second half of 2025, Batavia Biosciences is adding commercial manufacturing services for its clients in its new commercial production facility. The company has worked with renowned CROs that offer high-quality test models and toxicology. It also provides CTOs that aid with the design and execution of clinical trials. It provides its clients with an end-to-end solution for developing biopharmaceutical products through its global network and complete product development package. Its vision is to reduce human suffering by contributing substantially to increased accessibility and global affordability of state-of-the-art biopharmaceutical medicines.
Providing Solution to its Clients
Batavia Biosciences acts more like a thought partner to its clients, unlike under-contract development manufacturing organizations (CDMO). The combination of expertise, innovative solutions, technology platforms, and biological materials enables the company to guide clients toward success with great speed and precision. For instance, the company has developed technologies that tackle the three major issues encountered in the field, i.e., product yield (STEP® technology) and scale-up (SCOUT® technology & HIP-Vax® technology). In terms of biological materials, it can offer its clients diverse viral vector systems or virus seeds, and as well the proper mammalian cell line for manufacturing. As such, it provides a one-stop-shop solution to its clients.
As management, Batavia Biosciences always carefully listens to its clients to understand their needs and to translate that know-how into improved customer service offerings. For instance, Batavia Biosciences learned that founders/CEOs of start-up companies often face challenges in overseeing the entire process, including the risks and pitfalls, when moving an innovative new medicine from bench to clinic to market. Hence, it decided to roll out its ‘Product Development Plan’ (PDP) services package. Herewith, it is offering a customer-specific package that includes a full-fledged business plan.
This business plan is designed to help founders and CEOs address the various questions they may encounter from both internal and external stakeholders. The PDP plan is an all-encompassing plan describing the product, the business assumptions, the manufacturing and scale-up phases from pre-clinical to commercial, the clinical and regulatory pathways, and IP and communication strategies.
Overcoming Obstacles
Along with the cofounder, Dr. Chris Yallop, Menzo deployed a modest 300 square meters of R&D laboratories and only 7 people staff when the company was established. Through the network, quality, and vast experience of its staff, they were able to attract biotech and pharma clients. This pressured them as management to diligently expand the infrastructure footprint as well as the team. Batavia Biosciences was fortunate to be hosted by the TNO organization during its launch.
TNO demonstrated great flexibility by accommodating the company’s need to expand its laboratory infrastructure not just once, but three times within two years. The company made individual hires and acquired Xendo Pharma Services BV in 2011, just one year after its launch. These strategic actions proved to be crucial in expanding the team. The acquisition of Xendo Pharma Services BV brought in 117 highly trained professionals, significantly bolstering the company’s workforce. Within two years, the team grew to approximately 25 full-time equivalent (FTE) employees.
The next challenge came about in 2012 when Batavia Biosciences decided to embark on two major expansions simultaneously: the launch of a subsidiary in Boston, USA, and the addition of GMP manufacturing services in Leiden, the Netherlands. These decisions were again driven entirely by client demand.
Revenue Growth & Statistics
Batavia Biosciences experienced significant revenue growth, going from approximately 1 million Euros at its launch in 2010 to about 7 million Euros in 2012. It became evident that adding Good Manufacturing Practice (GMP) manufacturing services was necessary to further expand the company’s operations. The decision to choose the latter option was primarily motivated by client demand. Clients expressed their need for the company to provide not only the development of a manufacturing process but also the capability to effectively transform it into the delivery of a clinical product.
Having contracts in place to deliver clinical products, the company was pushed to build a quality system, hire expert staff including QC (Quality control), QA (Quality assurance), and QP (qualified person), as well as secure GMP manufacturing space. The company successfully launched its first clinical products. To facilitate this, they utilized a nearby GMP facility that could be leased on a per-project basis. This arrangement allowed the company to meet the immediate need for manufacturing capabilities without having its own facilities in place. Without a doubt, the decision to expand services from R&D only to GMP manufacturing spurred both client interest as well as organizational growth. By the end of 2013, the company employed about 55 FTE staff.
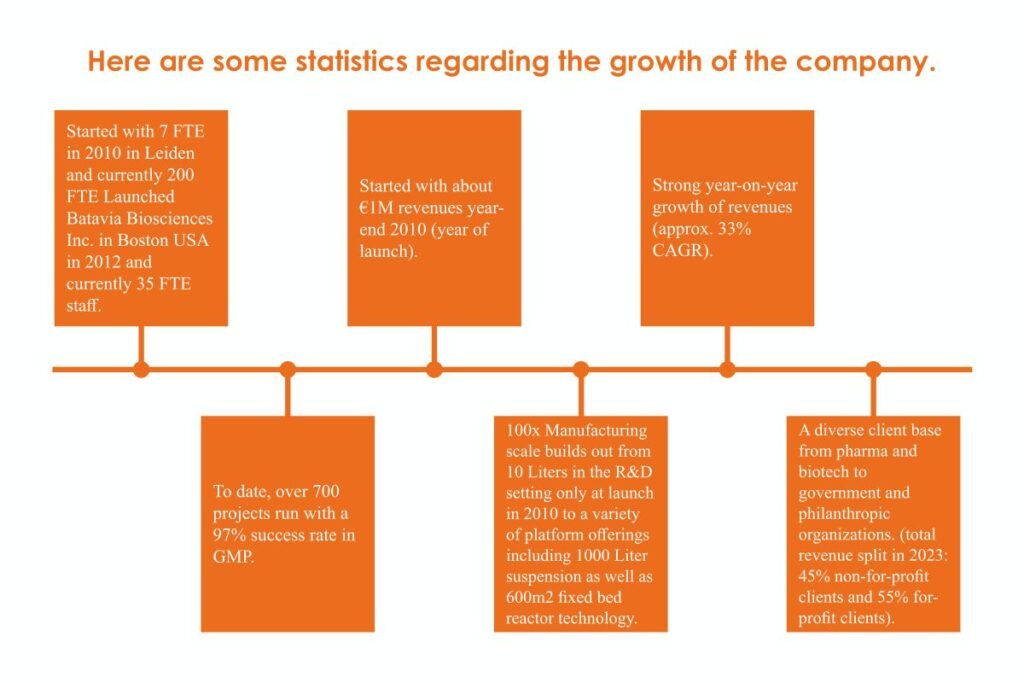
Here are some statistics regarding the growth of the company.
- Started with 7 FTE in 2010 in Leiden and currently 200 FTE Launched Batavia Biosciences Inc. in Boston USA in 2012 and currently 35 FTE staff.
- Started with about €1M revenues year-end 2010 (year of launch).
- Strong year-on-year growth of revenues (approx. 33% CAGR).
- To date, over 700 projects run with a 97% success rate in GMP.
- 100x Manufacturing scale builds out from 10 Liters in the R&D setting only at launch in 2010 to a variety of platform offerings including 1000 Liter suspension as well as 600m2 fixed bed reactor technology.
- A diverse client base from pharma and biotech to government and philanthropic organizations. (total revenue split in 2023: 45% non-for-profit clients and 55% for-profit clients).
Standing Out from Others and Path to Success
Menzo credits the company’s staff for its long-standing success. Their hard work and dedication make a difference for their clients. The company has always been awarded by a high percentage of returning clients. It has adopted the customer intimacy business model that aims to build durable relationships with its clients based on trust and quality. The staff’s experience is critical in finding effective scientific solutions to move innovative novel medicines from bench to clinic. Menzo believes that, as management, working on projects to support global health is an important intrinsic motivator for their staff.
To distinguish oneself from competitors, start by mapping your competitors and understanding the level of competition. For instance, Batavia Biosciences’, as a center of excellence, is not a price fighter and therefore many of the CDMO players that offer low-end, cost-effective repetitive services are not in competition with the company. It offers value-added services through its experience, biological materials, and technology platforms and as such, there are not many players with the same track record that it has built. Becoming a one-stop-shop for our clients, ie, R&D clinical and commercial services, ensures that our clients will stay with us and that provides a solid basis to keep growing the company
Company Culture & Employee Support
Batavia Biosciences’ employees are the key to its growth and it’s their experience and dedication that allows it to exert the customer intimacy client model. Next to ensuring competitive remuneration, it firmly believes that job satisfaction is crucial to binding talent to the company. It translates job satisfaction into exciting work projects that aim, for instance, to make vaccines more affordable, protect humans against deadly pathogens, or substantially increase the quality of life for humans suffering from hereditary genetic defects.
Apart from that, flexibility and career planning are also crucial in being a solid partner for its employees. It supports the staff wishing to make a career move, or in changing geographical location. The management team is very active as employers to help their employees via training to expand their skills.
Belief and Leadership Philosophy
Menzo believes in the concept of “Plan your dive and dive your plan”. This means that once the entrepreneur has convinced himself/ herself to spend all the energy available to make a success of one’s commercial dream that he/ she should stay focussed on building that success. Many entrepreneurs fail as they lose focus too early. He deems it critical for an entrepreneur to find the right people to follow the dream, which requires a well-thought-out, externally validated, and realistic business plan. In addition, being honest about the risks and rewards to build win-win situations ensures that you will be able to attract the necessary supporters when moving from start-up to scale-up.


